|
Sung-Hou
Kim, Principal Investigator and Component Project Leader
 |
 |
|
|
| |
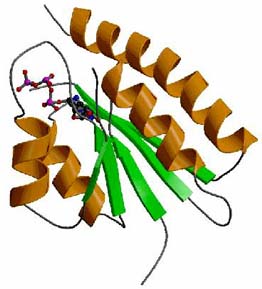
The
crystal structure of MJ0577, a "hypothetical protein" from Methanoccocus
jannaschii, which has no sequence similarities to any proteins
of known functions. The structure revealed a new protein fold
and a new ATP binding motif. Biochemical tests of its molecular
function inferred by the structure suggest that the protein
is a molecular switch that hydrolyzes ATP in the presence of
its partner proteins. |
| |
|
BSGC
Role
Sung-Hou Kim is the principal investigator of the BSGC.
He will be coordinating the entire project and overseeing the Component
Project III involving the structure determination
of the proteins purified by Component II by X-ray crystallographic methods.
Component Project
Information
Analysis of several
genomic sequences indicates that no known functions can be assigned
to a significant fraction of the genes. Since the molecular (biochemical
and biophysical) function of a gene product is tightly coupled to
its three-dimensional structure, finding the structure or its folding
pattern may provide important insight into the molecular function
of the gene product. That, in turn, may help in understanding its
cellular function (networks of many molecular functions) as well.
We propose to construct a comprehensive structural representation
of most of the gene products of two of the simplest minimal organisms, the human pathogens
Mycoplasma genitalium and Mycoplasma pneumoniae. We do this by determining the three-dimensional
structures by X-ray crystallography of the fold representatives of
most of the proteins in these organisms or their homologs from
other organisms. The structures will be computationally analyzed,
and, when the structures implicate possible molecular functions, they
will be tested experimentally. These molecular functions and structural
information will be correlated with the cellular function of the gene
products.
Furthermore, we plan to map these structures on a global protein fold space to understand the structural phylogeny.
|

|